Free Shipping on Orders Over $500! Shop Now>>
INDICAID COVID-19 Rapid Antigen Test
$30.00 – $375.00
INDICAID COVID-19 Rapid Antigen Test
Manufacturer: Phase Scientific
INDICAID COVID-19 Rapid Antigen Test
Manufacturer: Phase Scientific
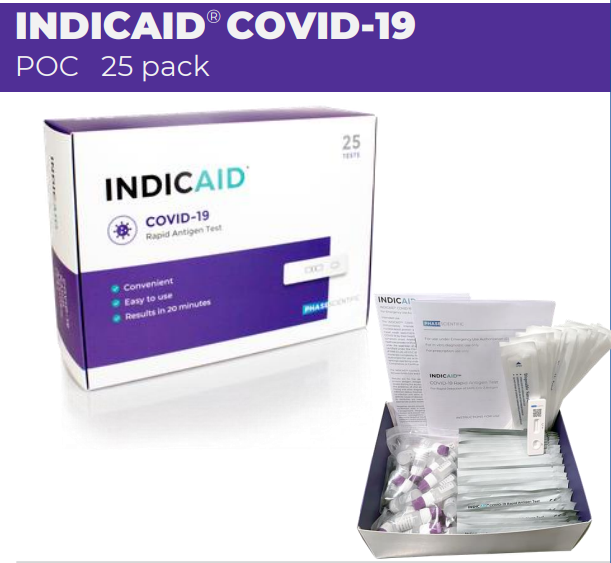
Packaging: Box (25 Tests), Case (450 Tests)
$30.00 – $375.00
The INDICAID POC COVID-19 Rapid Antigen Test is a CLIA-waived, quick, and reliable tool for detecting COVID-19 in just 20 minutes. (Short Dated – Expires July 6, 2025)
Shipping Options:
- Ground
- 2-Day
- Next Day
Urgent Delivery: Need this product urgently? Call us now! If you require this product on a tight deadline or have urgent delivery needs, please give us a call at (407) 813-2442
Product Description
The INDICAID COVID test offers a lateral flow immunoassay designed to detect the nucleocapsid protein antigen from SARS-CoV-2. Suitable for healthcare professionals, it provides results in 20 minutes, ensuring prompt decision-making for patient care. Its ease of use and rapid turnaround make it ideal for busy clinical settings.
Phase Scientific INDICAID COVID-19 POC Rapid Antigen Test Key Features
- Rapid results in 20 minutes
- Easy to use with minimal training required
- High sensitivity and specificity for accurate detection
- Suitable for various point-of-care settings
- No specialized equipment needed
These features ensure that healthcare providers can perform testing quickly and efficiently, helping to identify and manage COVID-19 cases effectively.
What’s Included in the Phase Scientific INDICAID Point of Care Rapid Antigen Test
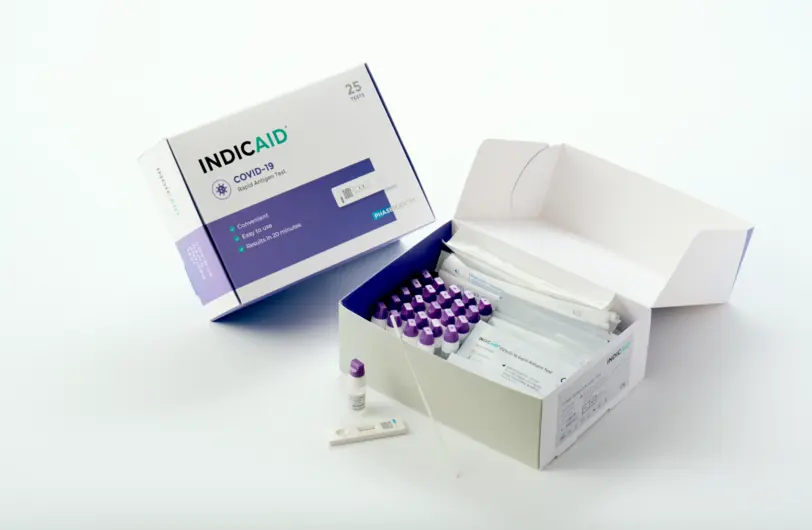
The INDICAID Point of Care Rapid Antigen Test kit includes all the necessary components to perform the test accurately and efficiently. The kit is designed for ease of use, ensuring healthcare providers have everything they need.
- 25 INDICAID test devices
- 25 sample collection swabs
- 25 reagent solution vials
- 1 package insert with detailed instructions
- 1 quality control test set
These items ensure that each test can be performed with precision and reliability, allowing healthcare professionals to be confident in the results.
Following the INDICAID COVID-19 Test Instructions
Using this COVID-19 rapid test is straightforward and can be completed in just a few steps:
- Step 1 – Prepare the Kit: Unbox the kit and lay out its components, including the test device, buffer solution, and swab.
- Step 2 – Collect the Sample: Collect the nasal swab sample carefully following the test instructions
- Step 3 – Mix the Sample: Mix the sample with the reagent solution in the provided vial.
- Step 4 – Apply Drops: Apply a few drops of the solution to the test device.
- Step 5 – Read the Results: Wait 20 minutes and read the results based on the clear indicators.
Where to Buy the Phase Scientific INDICAID Point of Care (CLIA WAIVED) Rapid Antigen Test
Purchase this rapid test from GSE Medical Supplies for competitive pricing, fast shipping, and reliable customer service. Bulk purchase options are also available to accommodate larger healthcare facilities.
Resources
Product Details
Additional information
| Weight | 18 lbs |
|---|---|
| Dimensions | 20.5 × 18.8 × 10 in |
| Manufacturer | Phase Scientific |
| Packaging | Box (25 Tests), Case (450 Tests) |
| Brand | Indicaid |
Resources
Safety Data Sheet
Package Insert
Watch this video to see how MedMira’s patented Rapid Vertical Flow Testing is transforming point-of-care testing.
Frequently Bought Together
Frequently Bought Together
-

Naloxone HCl Nasal Spray 4mg
Rated 2.80 out of 5Price:Add to cart$42.99Original price was: $42.99.$32.99Current price is: $32.99. -

INDICAID Rapid Strep A Antigen Test
Rated 2.00 out of 5Price:Add to cart$39.99
-

Molnlycke Hibiclens Antiseptic Skin Cleanser
Price:Select options This product has multiple variants. The options may be chosen on the product page$8.99 – $936.00
-

3M 1860 N95 Mask
Price:Select options This product has multiple variants. The options may be chosen on the product page$38.35 – $230.14
FAQs
What does GSE Medical Supplies offer?
GSE Medical Supplies provides a wide range of medical products, including wound care, diabetes management, incontinence supplies, diagnostic tests, and more. Their goal is to improve procurement efficiency and patient outcomes through high-quality healthcare solutions.
What is the accuracy of the INDICAID COVID-19 Rapid Antigen Test?
The INDICAID COVID-19 Rapid Antigen Test offers high sensitivity and specificity, ensuring reliable detection of active COVID-19 infections.
How long does it take to get results?
Results are available within 20 minutes, making it a fast and efficient option for point-of-care testing.
Are the INDICAID COVID-19 tests easy to use?
Yes, the test is designed for ease of use, with detailed instructions included in the kit for accurate testing.
Can the INDICAID COVID-19 test be used for asymptomatic patients?
Yes, the test can detect COVID-19 in both symptomatic and asymptomatic individuals. For high-suspicion cases, confirmatory PCR testing may be recommended for negative results.
What does the INDICAID COVID-19 Rapid Antigen Test kit include?
The kit contains test devices, swabs, reagent vials, and clear instructions, providing all the tools needed for accurate and efficient testing.
Who manufactures the INDICAID COVID-19 Rapid Antigen Test?
The test is manufactured by Phase Scientific International, a biotechnology company committed to high-quality diagnostic solutions.
What does GSE Medical Supplies offer?
GSE Medical Supplies provides a wide range of medical products, including wound care, diabetes management, incontinence supplies, diagnostic tests, and more. Their goal is to improve procurement efficiency and patient outcomes through high-quality healthcare solutions.
Who are the partners of GSE Medical Supplies?
GSE Medical Supplies partners with well-known brands like Medline, McKesson, Henry Schein, NDC, and NorthShore Care. They also have their own manufacturing partners to offer a diverse range of products.
Is GSE Medical Supplies a division of Global Supply Exchange?
Yes, GSE Medical Supplies is a division of Global Supply Exchange, focused specifically on providing top-tier medical supplies and equipment to healthcare professionals, businesses, and consumers.
Does GSE Medical Supplies have any government contracts?
Yes, GSE Medical Supplies has supplied products to government agencies like the US Navy, Department of Justice, and FDA. They have fulfilled large-scale orders, such as millions of COVID-19 tests for various CMS programs.
What makes GSE Medical Supplies different from other suppliers?
GSE Medical Supplies focuses on delivering high-quality, reliable products with a commitment to excellent customer service and fulfillment capabilities. As a women-owned business, they also emphasize partnerships and meeting the specific needs of their clients, including B2B and B2C fulfillment services.
How quickly does GSE Medical Supplies ship product?
GSE Medical Supplies aims to ship products quickly and efficiently, typically processing and shipping orders within 1-2 business days. Shipping times may vary depending on the product and destination, but they prioritize prompt delivery to meet customer needs. For specific shipping details, customers can contact GSE Medical Supplies directly to inquire about their order and expected delivery timelines.
Is Global Supply Exchange a women-owned business?
Yes, Global Supply Exchange is a women-owned business and has established a strong track record of working with government organizations and delivering essential healthcare products.
Does GSE Medical Supplies offer discounts on bulk purchases?
Yes, GSE Medical Supplies offers bulk purchase discounts. The qualifications for a bulk purchase may vary depending on the specific product category and order size. Generally, larger quantity orders of medical supplies, such as pallets of incontinence products, diagnostic tests, or wound care supplies, are considered bulk purchases. For precise details on what qualifies as bulk and the discount rates, customers should contact GSE Medical Supplies directly to discuss their specific needs.
Does Global Supply Exchange offer fulfillment services?
Yes, Global Supply Exchange provides fulfillment services, handling both B2B and B2C shipments, and has fulfilled millions of orders for government programs, such as COVID-19 test kits for CMS initiatives.
Trusted by over 20,000 customers

Testimonials
About Global Supply Exchange
GSE Medical Supplies, a division of Global Supply Exchange, is a leading distributor of high-quality medical supplies and lab solutions, serving over 500 healthcare clients. We specialize in a wide range of products, including diagnostic instruments, protective apparel, skin and wound care, and point-of-care lab solutions.
As a member of the NuEdge Alliance and a registered NDC/NuEdge GPO distributor, we leverage group purchasing programs like Vizient and Provista to offer competitive pricing.
Our commitment to fast shipping, exceptional customer service, and affordable, reliable products supports healthcare professionals in delivering superior care. Established in 2020 as a Women-Owned Business, we continue to meet the evolving needs of the medical industry.